Electrochemical Patterning of Metals
Electrochemical Patterning and Functionalization of Metal surfaces/Scratch-Resistant Antibiofouling Surfaces
The electrochemical treatment of metals is a well-established industrial process. Well-known examples are the anodization of aluminum and titanium alloys as well as the passivation of stainless steels.
This treatment leads to the formation of metal oxides at the surface that impart higher corrosion resistance and higher surface hardness. Depending on their structure and chemistry, the oxide layers may also constitute a diffusion barrier to metal ions from beneath the surface.
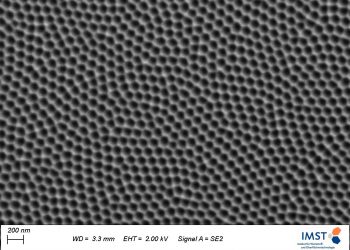
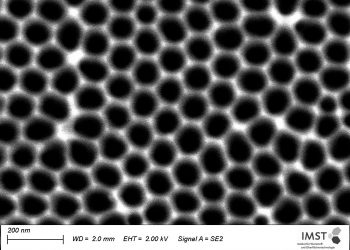
A little less known is the surface patterning via electrochemical treatment. That means generating well ordered (nano)structures on the surface. Of particular interest are ordered porous structures with tunable pore diameter, pore wall thickness and pore length. These pores are generated via a self-ordering that involves specific electrochemical reactions at the electrolyte-metal/oxide interface.
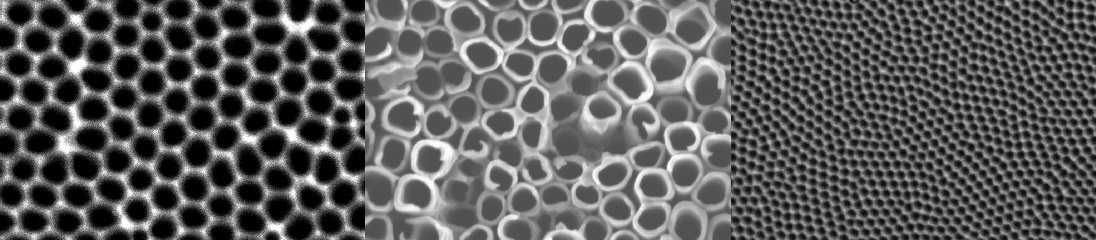
Scanning electron micrographs of electrochemically patterned surfaces of a titanium alloy (left), aluminum (middle) and stainless steel (right). Pore size: 100 nm.
At IMST, electrochemically patterned metals (titanium, aluminum, steels, including carbon steels) are used as a universal platform for different applications.
Some examples of the applications that were developed at IMST:
- Metal surface patterning for achieving a high adhesion strength of metal-polymer composites and metal-adhesives
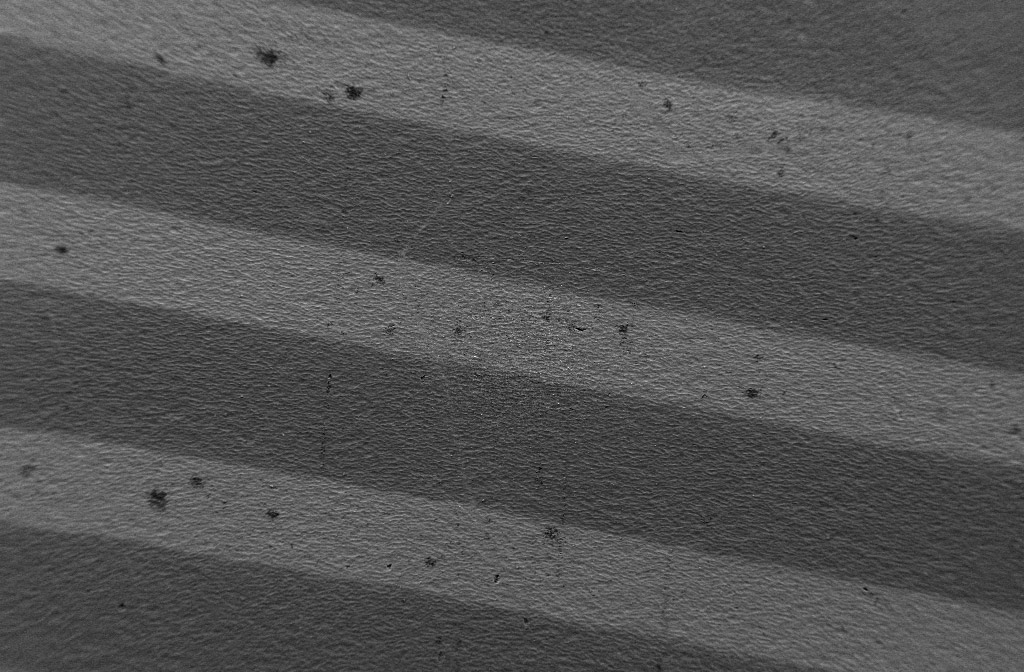
- 3 dimensional (3D), scratch-resistant antibiofouling layers (EP 18703549.8, granted, inventor Es-Souni): An antibiofouling layer is photo-grafted on the surface and pore walls of electrochemically structured metals (aluminum, titanium, steel etc.). The porous oxide layer endows the polymeric antibiofouling thin film with the necessary mechanical resistance it lacks on flat surfaces. Implant for traumatology as well as submerse maritime surfaces can be functionalized with such scratch-resistant antibiofouling layers (see pictures below)
A titanium alloy surface after electrochemical anodization and photografting of an anti-biofouling polymer. The surface has been patterned in functionalized (dark ) and non-functionalized stripes (bright) to better show the anti-biofouling effect. Bacteria adhere only on the bright stripes: The dark patches are biofilms that are magnified in the right image.
Anti-Fog and Self-Cleaning Surfaces
- Anti-fog and self-cleaning surfaces for fittings and metal facings: The electrochemical patterning amplifies the hydrophilic viz. Hydrophobic character of the top-layer. This results in so-called superhydrophilic viz. superhydrophobic surfaces that are self-cleaning. The video below shows and example where water spreads on a titanium superhydrophilic surface with a water contact angle of 0°
A water drop spreads on a titanium surface that was made superhydrophilic via a combination of electrochemical treatment and polymer photografting. A 7 µm thick water film forms on the surface.